On 22nd November 2023, the Department of Health and Social Care published its full response to the review into commercial clinical trials.
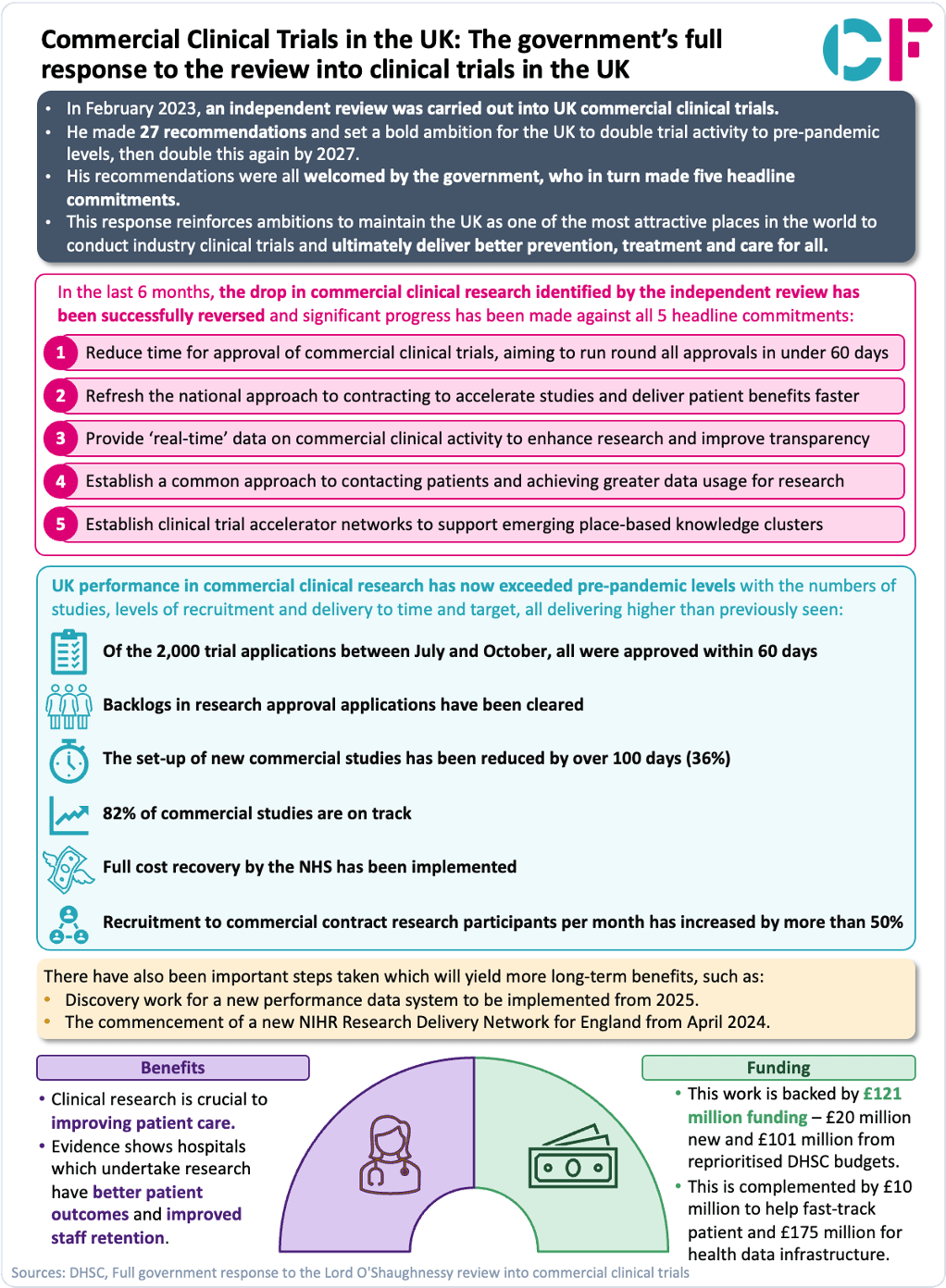
The response outlines plans to make the UK one of the best places in the world to conduct clinical trials by implementing recommendations from an independent review, following an announcement in May this year to speed up clinical trials and make it easier for revolutionary healthcare treatments to get to NHS patients, backed by £121 million in government funding.
In the last six months we have seen a reversal of the drop in commercial clinical research identified by the review and made significant progress against all five of the government’s headline commitments. UK performance in commercial clinical research has now exceeded pre-pandemic levels with the numbers of studies, levels of recruitment and delivery to time and target, all delivering higher than previously seen.
This response outlines ambitions to maintain the UK as one of the most attractive places in the world to conduct industry clinical trials as well as progress made to date to achieve this.
Our latest snapshot provides a summary of this response :
Click here to access the full response.




